Parliament Passes Bills for Rationalisation of Gender and Special Interest Groups
Ayota remains NSSF MD after court dismisses Byarugaba case
Former NSSF MD Patrick Byarugaba loses case seeking contract renewal
UGANDA U16 WOMEN’S TEAM ENDS UEFA FRIENDSHIP TOURNAMENT EXPEDITION ON A HIGH
Chinese firm petitions Parliament over Kilembe Mines
Parliament Passes Bills for Rationalisation of Gender and Special Interest Groups
Ayota remains NSSF MD after court dismisses Byarugaba case
Former NSSF MD Patrick Byarugaba loses case seeking contract renewal
UGANDA U16 WOMEN’S TEAM ENDS UEFA FRIENDSHIP TOURNAMENT EXPEDITION ON A HIGH
Chinese firm petitions Parliament over Kilembe Mines
MPs reject move to rationalise National Forestry Authority
Parliament approves rationalisation of gov’t agencies
City traders close shops in protest
Kanyamunyu free after serving 3 years and 5 months in prison
Thugs break into Luwero CAO, RDC and LC5 offices

President Museveni Promotes Sam Kavuma, Assigns Him Somalia Task

UN court to deliver ruling on South Africa’s genocide case against Israel

M23 appoints former DRC electoral commission chairman alliance coordinator
Army officers say they are taking power in Gabon
Botswana submits bid to host AFCON 2027

Explosions as Ugandan forces battle militants in Somalia
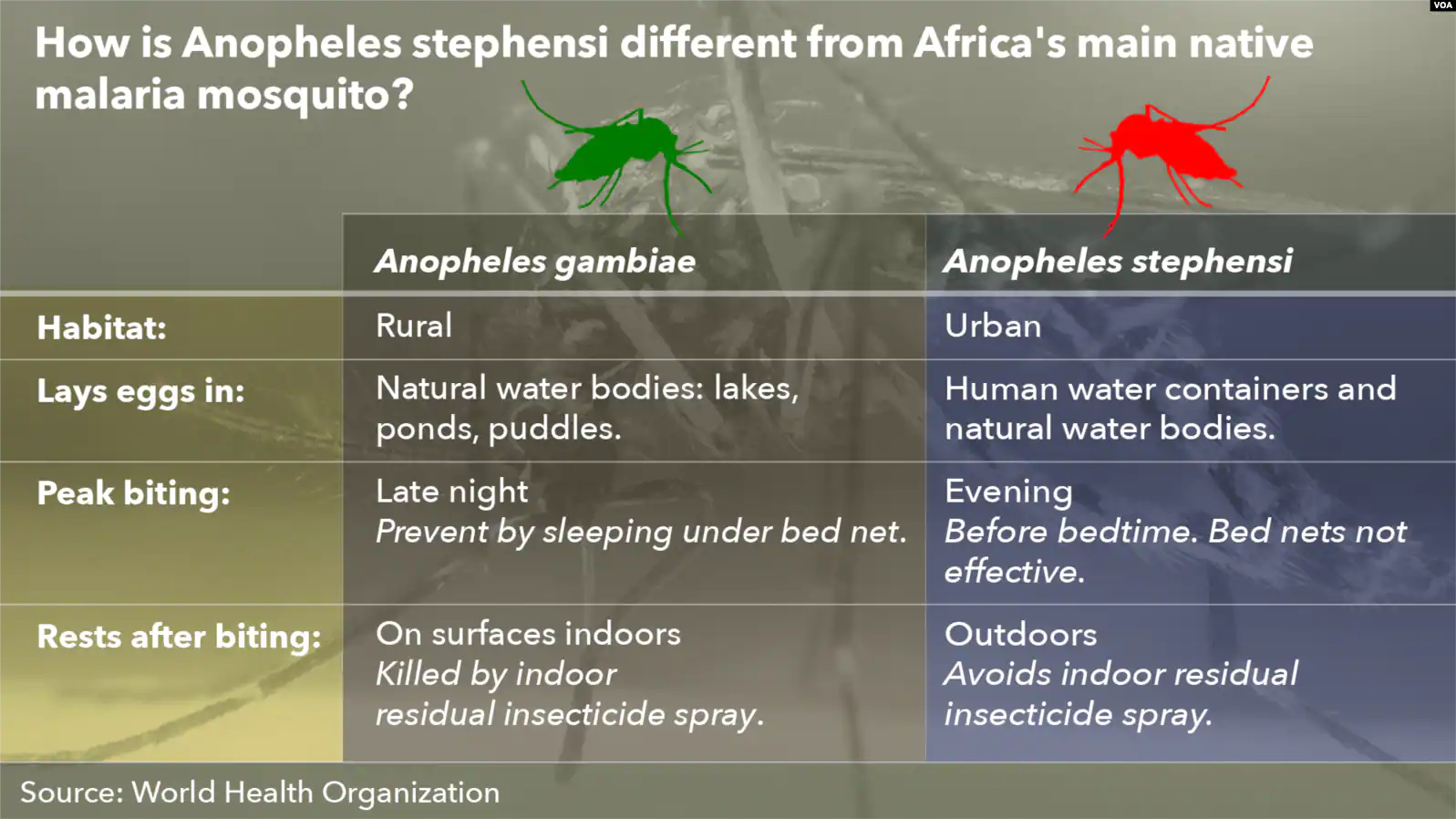
Invasive Mosquito Threatens Malaria Control in Africa

Mali Rights Commission Condemns Restrictions on Free Press

EAC SUMMIT: Rwanda’s Kagame meets DRC Prime Minister

Queen Elizabeth II: A life in pictures
UGANDA U16 WOMEN’S TEAM ENDS UEFA FRIENDSHIP TOURNAMENT EXPEDITION ON A HIGH
by Manager - Innerman Radio | April 19, 2024 | Local News, Sports News | 0 Comments
CAF questions quality of Namboole stadium renovation
by Manager - Innerman Radio | April 12, 2024 | Business News, Sports News | 0 Comments
Reconsider phasing out of nursing courses’
by Manager - Innerman Radio | April 3, 2024 | Business News, Health, Sports News | 0 Comments
UNEB Releases UCE Sample Examination Papers Under New Curriculum
by Manager - Innerman Radio | March 25, 2024 | Local News, Sports News | 0 Comments
Makerere named among top 10 universities in Sub-Saharan Africa
by Manager - Innerman Radio | June 26, 2023 | Local News, Sports News | 0 Comments
Botswana submits bid to host AFCON 2027
by Manager - Innerman Radio | May 26, 2023 | International News, Sports News | 0 Comments
Gov’t Sports Bill Proposes Tough Penalties for Sexual Exploitation, Results Manipulation
by Manager - Innerman Radio | December 12, 2022 | Local News, Sports News | 0 Comments
14000 Examiners deployed to mark P7, S4 exams – UNEB
by Manager - Innerman Radio | November 29, 2022 | Local News, Sports News | 0 Comments
Religious leaders oppose govt’s move to close PTCs
by Manager - Innerman Radio | November 28, 2022 | Politics, Sports News | 0 Comments
AFCON U20 Zonal Qualifiers: Hippos determined to seal qualification against hosts Sudan
by Manager - Innerman Radio | November 8, 2022 | Local News, Sports News | 0 Comments
Ayota remains NSSF MD after court dismisses Byarugaba case
by Manager - Innerman Radio | April 19, 2024 | Business News, Local News, Politics | 0 Comments
Former NSSF MD Patrick Byarugaba loses case seeking contract renewal
by Manager - Innerman Radio | April 19, 2024 | Business News, Local News | 0 Comments
CAF questions quality of Namboole stadium renovation
by Manager - Innerman Radio | April 12, 2024 | Business News, Sports News | 0 Comments
BOU asks URSB to make changes to Crane Bank name
by Manager - Innerman Radio | April 4, 2024 | Business News, Local News, Politics, Security | 0 Comments
Reconsider phasing out of nursing courses’
by Manager - Innerman Radio | April 3, 2024 | Business News, Health, Sports News | 0 Comments
‘Move staff between projects to save costs’
by Manager - Innerman Radio | April 3, 2024 | Business News, Local News, Politics | 0 Comments
Local council Elections Extended Another 180 Days.
by Manager - Innerman Radio | January 11, 2024 | Business News, COVID-19, Local News, Politics, Security | 0 Comments
Gov’t to stop paying for unused electricity
by Manager - Innerman Radio | January 9, 2024 | Business News, Local News | 0 Comments
Prepaid Yaka electricity meters to be phased out by November 2024
by Manager - Innerman Radio | January 8, 2024 | Business News, Local News, Security | 0 Comments
BUSOGA BUSINESS LEADER PAYS COURTESY CALL TO URA
by Manager - Innerman Radio | January 8, 2024 | Business News, Local News | 0 Comments
Parliament Passes Bills for Rationalisation of Gender and Special Interest Groups
by Manager - Innerman Radio | April 22, 2024 | Local News, Politics, Security | 0 Comments
Ayota remains NSSF MD after court dismisses Byarugaba case
by Manager - Innerman Radio | April 19, 2024 | Business News, Local News, Politics | 0 Comments
Chinese firm petitions Parliament over Kilembe Mines
by Manager - Innerman Radio | April 19, 2024 | Local News, Politics, Security | 0 Comments
Parliament approves rationalisation of gov’t agencies
by Manager - Innerman Radio | April 19, 2024 | Local News, Politics, Security | 0 Comments
Thugs break into Luwero CAO, RDC and LC5 offices
by Manager - Innerman Radio | April 12, 2024 | Local News, Politics, Security | 0 Comments
Junior civil servants tell IGG why they are reluctant to report corruption cases
by Manager - Innerman Radio | April 5, 2024 | Local News, Politics, Security | 0 Comments
Gen Mbadi ready to sweep mess at UNBS, Trade Ministry
by Manager - Innerman Radio | April 5, 2024 | Local News, Politics, Security | 0 Comments
BOU asks URSB to make changes to Crane Bank name
by Manager - Innerman Radio | April 4, 2024 | Business News, Local News, Politics, Security | 0 Comments
Govt to investigate fraudulent land dealings in Agago district
by Manager - Innerman Radio | April 3, 2024 | Local News, Politics, Security | 0 Comments
‘Move staff between projects to save costs’
by Manager - Innerman Radio | April 3, 2024 | Business News, Local News, Politics | 0 Comments
Stevour Chritian High School
Stevour Christian High School is founded on grounds of answering a calling of a long time great need for a school that will bring out products of students who excel academically, prosper in life but most importantly having Christ central at heart, character, virtues, benefiting the society and aiming at eternal life. Click for more
Innerman Ministries - UCC
United Christian Centre (UCC) is a registered Pentecostal Church in Makerere Kikoni – Kampala and the home of Innerman Ministries International. UCC Church is being pastored by Pastor Dr. Joseph Selumaga a ministry successor to Bishop Stephen Senfuma who the Lord called back to himself in 26th June 2021.
Innerman Primary School
Innerman Pre-Primary School is a Church founded school that was setup in order to address the high illiteracy levels in the community that was caused due to heightened academic costs in the region. for more information Call +256707222888 or +256783671883.